Lavoisier classification of elements
In the beginning of 19th century Lavoisier classified elements to Metals and Non metals.
But this classification is not enough to study large number of elements in easy way.
So, to make it more simpler there was several attempts .
Dobereiner's triads
Johann_Wolfgang Dobereiner
In 1829 German chemist noticed that there is a relationship between atomic weight and properties of elements.
He make a group of three elements having similar properties, he arranged them in such a manner that the arithmetic mean of mass of first and third element is equal to the mass of middle one.
Examples of some triads
Achievements of Dobereiner triads
Dobereiner achieved to build a relationship between properties of elements and atomic mass.
Limitations of Doberieners triads
At the time of doberiener only 30 elements were known but he was unable to fit all the elements into triads. He could able to make just three triad's.
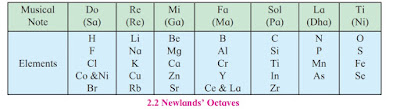
Newland's Octaves
In 1863 British Chemist John Newland proposed a new system of grouping of elements.
According to him, If elements were arranged according to increasing atomic weight then property of every eight element is similar to the first element just like a musical note.
For example
Achievement of NewLand's Octaves
This is a better way to grouping elements. In this arrangement triads of doberiener can be seen.
In this way more number of elements can be classifieds into groups.
Limitations of Newlands Octave.
- Newland arranged all 56 known elements in his classification but his law of octave is valid up to calcium (mass=40) only.
- New discovered elements (having small mass) cannot be placed in his classification.
- He just make a sequence of elements, he didn't make any table or boxes.
Lother Mayer's Arrangement
In 1869 a German chemist Lother Mayer plotted a graph between atomic mass and atomic volume. He noticed that elements having similar properties occupies the same position in his graph. This arrangement is accurate and matched today's modern periodic table. But still it was lacking.
Lacking of this arrangement
It's too tough to remember and explain the positions of elements.
Mendeleev's periodic Table
In 1869 a Russian scientist D.I Mendeleev classified elements in a systematic manner.
He arranged elements according to his law.
Mendeleev's periodic law
"Chemical and physical properties of elements are periodic functions of their atomic masses."
Mendeleev arranged all the known 63 elements in the form of table. This classification has vertical coloums called Group and horizontal rows called periods.
Structure of Mendeleev's periodic table
Mendeleev kept elements in boxes, according to increase in atomic mass. In the top of group he has written the general formula of their oxides and hydrides.




0 comments:
Post a Comment
Please do like share and subscribe our blog